Clinical trials area
Promoting clinical research is one of the primary objectives of the IIS-FJD, as investigation is a fundamental tool for the development and application of new measures to further prevention, diagnosis, and therapy in clinical work and make progress toward identifying and solving the limitations of existing procedures. Of all types of research, randomized controlled clinical trials are the best method available to assess the efficacy and safety of new interventions.
Since it was first accredited as a Health Research Institute in 2010, the IIS-FJD has worked to promote quality clinical research and foster more efficient research management.
To do this, a number of measures have been enacted by the different groups involved:
- IIS Scientific Director:
- In order to benefit from external oversight of clinical research, we joined the BEST Project in 2013. Promoted by the pharmaceutical industry, this initiative seeks to bring together all of the stakeholders of clinical research—both public and private—and create a platform of excellence in clinical research of drugs within Spain. It aims to foster R& investment by objectively evaluating and monitoring the processes surrounding clinical research in Spain; identifying differences in practice; and taking the attendant measures so as to improve efficiency and make the industry more competitive.
- The initiative brokers contact between sponsors and medical doctors.
- The program follows-up on patient recruitment.
- Clinical Research Ethics Committee of the IIS-FJD: By putting the procedure of mutual recognition into practice when evaluating a clinical trial, the Ethics Committee honors the decisions of the reference ethics committee for multi-center clinical trials.
- Research Management Area:
- Simultaneous management of the evaluation of the trial protocol and contract, forms for which are adapted to the single contract used in the region of Madrid (available on the web—include link).
- Unification of the contract terms for the three health centers that operate under the aegis of the FJD Ethics Committee (Hospital UniversitarioFundación Jiménez Díaz, Hospital Universitario Infanta Elena, and Hospital Universitario Rey Juan Carlos).
- Internal monitoring of lead times for managerial tasks and patient recruitment.
RESULTS OF CLINICAL TRIALS
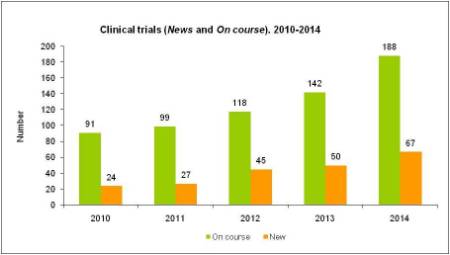 High resolution image. This link will open using lightbox, there may be a context switch
High resolution image. This link will open using lightbox, there may be a context switch